It’s only been eleven days since the start of 2024, and yet we have already received two calls from clients concerned about the state of their slabs and foundation walls. While concrete is a durable and versatile material that is widely used in homes and garages, concrete can also deteriorate over time due to various factors, such as corrosion of embedded metals, chemical attack, freeze-thaw cycles, abrasion, fire, shrinkage, overload, impact, loss of support and surface defects. In this blog post, we will discuss some of the most common types and causes of concrete deterioration and how to prevent them.
Corrosion of Embedded Metals (Most Common)

Corrosion of reinforcing steel and other embedded metals is the leading cause of concrete deterioration. When steel corrodes, the resulting rust occupies a greater volume than the steel. This expansion creates tensile stresses in the concrete, which can eventually cause cracking, delamination and spalling.
Steel corrodes because it is not a naturally occurring material. Rather, iron ore is smelted and refined to produce steel. The production steps that transform iron ore into steel add energy to the metal. Steel, like most metals except gold and platinum, is thermodynamically unstable under normal atmospheric conditions and will release energy and revert back to its natural state — iron oxide, or rust. This process is called corrosion.
For corrosion to occur, four elements must be present: There must be at least two metals (or two locations on a single metal) at different energy levels, an electrolyte (such as water or moisture), oxygen and a metallic connection (such as wire or rebar).
The most common causes of corrosion of embedded metals in concrete are:
- Chloride ions: Chloride ions can penetrate the concrete through cracks, pores or joints and reach the steel surface. Chloride ions can break down the protective layer of oxide that forms on the steel surface and accelerate the corrosion process. Chloride ions can come from deicing salts, seawater, groundwater or admixtures.
- Carbonation: Carbonation is the reaction of carbon dioxide in the air with the calcium hydroxide in the concrete. This lowers the pH of the concrete and reduces its alkalinity, which is essential for protecting the steel from corrosion. Carbonation can occur when concrete is exposed to air or when it has a low water-cement ratio.
- Dissimilar metal corrosion: Dissimilar metal corrosion occurs when two different metals are in contact with each other in an electrolyte. The more active metal (such as zinc or aluminum) will corrode faster than the less active metal (such as steel or copper). This can happen when different types of reinforcement are used in the same structure or when metal accessories are embedded in the concrete.
To prevent corrosion of embedded metals in concrete, some of the possible solutions are:
- Use corrosion-resistant reinforcement, such as stainless steel, epoxy-coated steel or galvanized steel.
- Use adequate concrete cover over the reinforcement to protect it from exposure to moisture, oxygen and chloride ions.
- Use low-permeability concrete with a high water-cement ratio and adequate curing to minimize cracking and pore size.
- Use admixtures or coatings that can inhibit corrosion or seal cracks.
- Avoid using deicing salts or other sources of chloride ions on concrete surfaces.
- Avoid mixing different types of metals in the same structure or use insulating materials to separate them.
- Install galvanic anodes (also referred to as sacrificial anodes) in contact with other reinforcement (such as steel rebar) to slow the onset of harmful corrosion.
Freeze-Thaw Deterioration (Very Common)
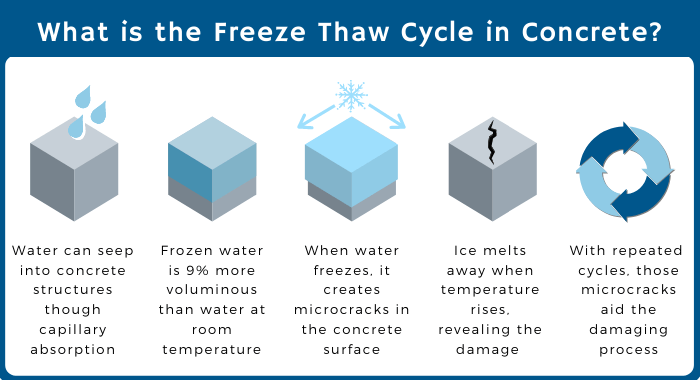
Freeze-thaw deterioration occurs when water freezes and expands inside the pores or cracks of concrete. This creates internal pressure that can exceed the tensile strength of the concrete and cause cracking, scaling or spalling. The most common causes of freeze-thaw deterioration in concrete are:
- Deicer scaling: Deicer scaling is the loss of surface mortar due to repeated cycles of freezing and thawing in the presence of deicing salts. Deicing salts lower the freezing point of water and increase the number of freeze-thaw cycles. They also increase the osmotic pressure inside the pores and draw more water into the concrete.
- Aggregate expansion: Aggregate expansion is the swelling of certain types of aggregates due to freezing and thawing. Some aggregates contain water-soluble minerals that can absorb water and expand when frozen. This can cause internal cracking or popouts in the concrete.
To prevent freeze-thaw deterioration in concrete, some of the possible solutions are:
- Keep moisture as far away from the concrete as possible. For foundations, this is accomplished by installing waterproof membranes against the walls. In garages, however, it is difficult to maintain the separation as vehicles bring in water through snow, ice and rain. Our recommendation is to brush off the vehicle to remove as much snow and ice a possible prior to entering the garage. We also recommend minimizing salt use around your home, and to make use of alternative products.
- Use air-entrained concrete, which contains microscopic air bubbles that can provide space for water to expand without causing damage.
- Use durable aggregates that are resistant to freezing and thawing and do not contain water-soluble minerals.
- Use proper finishing and curing techniques to avoid excessive bleeding, segregation or evaporation of water from the concrete surface.
- Avoid using deicing salts or other chemicals that can increase the risk of freeze-thaw damage.
Chemical Attack (Less Common)

Chemical attack is the deterioration of concrete due to exposure to aggressive chemicals that can react with the cement paste or the aggregates. Chemical attack can cause loss of strength, cracking, discoloration or erosion of the concrete.
The most common types of chemical attack on concrete are:
- Acids: Acids can dissolve the calcium hydroxide and the calcium silicate hydrate in the cement paste, which are the main components of concrete. Acids can also react with some aggregates and cause expansion or cracking. Acids can come from acid rain, industrial waste, fertilizers or organic matter.
- Salts and alkalis: Salts and alkalis can cause efflorescence, which is the formation of white crystals on the surface of concrete due to the migration and evaporation of water-soluble salts. Salts and alkalis can also cause alkali-silica reaction or alkali-carbonate reaction, which are discussed in the next section. Salts and alkalis can come from seawater, groundwater, deicing salts or cement.
- Sulfates: Sulfates can react with the calcium aluminate hydrate in the cement paste and form ettringite, which is a mineral that occupies more volume than the original components. This can cause expansion, cracking and spalling of the concrete. Sulfates can come from soil, groundwater, sewage or industrial waste.
To prevent chemical attack on concrete, some of the possible solutions are:
- Use low-permeability concrete with a high water-cement ratio and adequate curing to reduce the penetration of chemicals into the concrete.
- Use chemical-resistant admixtures or coatings that can protect the concrete from aggressive substances.
- Use appropriate types of cement and aggregates that are compatible with the exposure conditions and do not contain harmful substances.
- Avoid contact between concrete and sources of chemicals or provide adequate drainage and ventilation to prevent accumulation of chemicals.
Alkali-Aggregate Reactivity (Less Common)

Alkali-aggregate reactivity is a type of chemical reaction between the alkalis in the cement paste and certain types of aggregates that contain reactive silica or carbonate minerals. The reaction produces a gel-like substance that absorbs water and expands, causing internal pressure and cracking in the concrete.
The most common types of alkali-aggregate reactivity are:
- Alkali-silica reactivity: Alkali-silica reactivity occurs when the alkalis in the cement paste react with certain types of siliceous aggregates, such as chert, opal, flint or glass. The reaction produces a gel-like substance that absorbs water and expands, causing internal pressure and cracking in the concrete. The cracks are usually filled with the gel, which has a white or gray color.
- Alkali-carbonate reactivity: Alkali-carbonate reactivity occurs when the alkalis in the cement paste react with certain types of dolomitic aggregates, which contain both calcium carbonate and magnesium carbonate. The reaction produces a gel-like substance that absorbs water and expands, causing internal pressure and cracking in the concrete. The cracks are usually filled with the gel, which has a yellow or brown color.
To prevent alkali-aggregate reactivity in concrete, some of the possible solutions are:
- Use low-alkali cement, which has a lower content of sodium oxide and potassium oxide.
- Use non-reactive aggregates that do not contain reactive silica or carbonate minerals.
- Use pozzolanic materials, such as fly ash, slag or silica fume, which can reduce the alkalinity of the cement paste and consume some of the reactive silica in the aggregates.
- Use admixtures or coatings that can inhibit or control the reaction or seal the cracks.
